Morgan & Masterson Consulting
Medical Device Regulatory Submissions
Technological Aspects of Regulatory Submissions
For any medical device to be used in a patient, it has to be assessed by an appropriate regulatory authority. In the USA, this is the Food and Drugs Administration (FDA); in the European Union (EU), both individual member states and the European Medicines Agency are involved, while in the UK it is the Medicine and Healthcare Regulatory Authority (MHRA), in South Africa, the South African Healthcare Products Regulatory Agency (SAHPRA), in Japan by the Pharmaceuticals and Medical Device Agency (PMDA), in China by the National Medical Products Administration (NMPA), in Australia by the Therapeutic Goods Administration (TGA) and in Canada by Health Canada.
Many of these organizations have changed their structure and procedures radically within the last few years; most of these changes have been beneficial in terms of logic, but there are so many nuances related to regulatory approval that the processes involved in regulatory submissions, the maintenance of approvals and in monitoring adherence to them can be very complex. Most medical device companies of significant size have their own Regulatory Department, which is often tasked with oversight on quality control; indeed, most submissions to regulatory authorities for all but the highest risk products are dominated by adherence to quality control and risk management procedures. There are many consulting companies that provide expertise in these areas, but that is not where I am involved.
My experience and expertise is often sought in one of two respects; those situations, usually involving high risk implantable devices, where advice on how best to structure the technological parts of a submission, (rather than quality systems sections) is required, and, secondly, when submissions are not initially approved and where scientific, engineering and clinical input is necessary within the ensuing dialogue between company and regulator. A few anonymous examples may explain this.
Benefits and risks

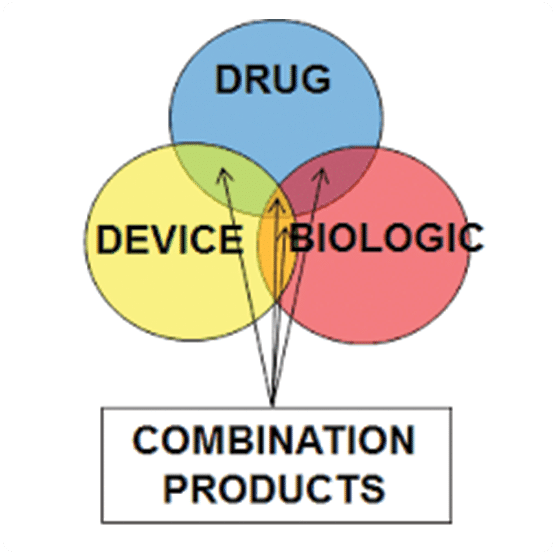
Classifications and borderline issues
Equivalence

Interactions with regulators

Morgan & Masterson Consulting
We offer global, high-level consulting for scientific and infrastructure aspects of cutting-edge medical technologies.
Our Expertise
Work with Us
We are commited to making a global impact and providing cost-effective solutions.



