Towards Clinical Trials of SAT Transcatheter Heart Valves
The technological challenges that we faced with this innovative program to develop heart valve replacements for young people suffering from RHD were considerable, but we were essentially ready to make submissions for implantation in humans by the beginning of 2020. This was later than we had hoped but we were confident of our success. By then, our laboratory and production facilities were very impressive. Crucially, our investors had backed us all the way. Our main investor was the Bidvest Group, a South African services, trading and distribution company, based in Johannesburg, who started providing equity funding in 2012. Successive funding agreements have allowed Bidvest, through further equity and loan arrangements, to maintain their support throughout. In addition, since the company is a start-up company derived from and based in The University of Cape Town, UCT have provided further equity investment in SAT.
Since then, a series of global disturbances, and one local difficulty, have delayed our clinical introduction, but we are now, towards the end of 2023, very close.
Despite the impact of COVID, global supply chain disruptions and skilled worker migration on “First in Man” (FIM) applications, our objectives to generate a technology platform that would be the basis of a global impact on RDH patients has stayed the course.
The Impact of COVID
The first ‘disturbance’, as everybody will appreciate, was the COVID-19 pandemic. Peggy and I were actually in South Africa in early 2020 when news of outbreaks of the viral infection were reported. Being due to return to the USA in March we were not unduly worried, but the increasing number of cases there in January and February, and the first case in South Africa reported on 5th March gave cause for concern. The rapid rise in internal transmission (as opposed to returning traveler transmission) persuaded the National Institute for Communicable Diseases to take strong actions, and our flight home on the 23rd March looked to be in serious jeopardy. Our flight from Cape Town to New York was the last to leave!
The impact on health-related activity in South Africa was profound. No elective surgery in our hospital at Groote Schuur; SAT as a medical company was allowed to continue, but under serious restrictions. Even as the pandemic started to ease, the last thing a large hospital suffering under huge constraints wanted to do was engage in new, unproven, surgical methods, however well-intentioned they were.
Global Supply Chains and Skilled Worker Migration
As months went by, we struggled to work on all of the verification testing of valve prototypes and other products, but then encountered a further consequence of the restriction on the global movement of goods. Our devices are technologically complex, requiring specialist materials and components that are often not readily available in Africa. An immediate consequence of COVID was that of supply chain disruption, and this affected us much more than with companies in Europe and North America This started as the imposition of much longer lead times and increased logistics costs, but then moved into phases of materials shortages and, ultimately unavailability. Being a small company, without international recognition and deep pockets, which happened to be located half a world away from most suppliers, caused many problems.
With critical medical device manufacture, choosing and finding alternative materials is not trivial since any significant material change (supplier, specification etc.,) means that all the previous validation testing may have to be repeated. In some situations, major international suppliers simply refused to deal with us, preferring their larger Northern Hemisphere customers.
Our highly skilled engineers, both in R & D and manufacturing phases, became adept at sourcing materials from other suppliers. However, the South African economy was under strain, and these same engineers found themselves vulnerable within the domestic environment and, one-by- one, they were enticed away to work in Europe for the companies with which they were now interacting. Nevertheless, we persevered, and still have a very competent workforce of around 60 people.
First in Man
Whenever a radically new medical device is ready to be evaluated in human patients, procedures encompassed by the phrase ‘First in Man’, or ‘FIM’, must be followed. The details vary from one jurisdiction to another. Within the Western Cape region of South Africa, we have to follow two essentially independent processes. One involves obtaining approval from the national authority to undertake a limited number of procedures on selected patients in order to demonstrate, as far as possible, the safety of the device; as seen before, that national authority is SAHPRA. The second involves obtaining approval from the Human Ethics Committee (HEC) of the hospital in which these first procedures will be undertaken, which is, in our case, Groote Schuur. Neither of these approval processes is easy or straightforward, nor should they be since the lives of the patients are at stake.
We recognized early on that there was an implicit conundrum within the ethical considerations related to our valve. The valves are intended for a population of young adults who have symptomatic RHD; they are not about to die, but their lives will be much shortened if they receive no treatment, and the quality of life while waiting to die will be poor. As with any invasive heart therapy, the procedure of implanting our valve will not be without risk. Either the procedure itself, or the valve, may produce irreparable harm of even death. The question is, therefore, how should the recipient of the FiM be selected? This is the most challenging ethical conundrum I have witnessed, but the choice could not be ours alone; the HEC had to decide on the principles of this choice. They decided not to allow us to operate on a young RHD patient, who, as I have stated, would not die immediately if they are not treated. Instead, they agreed that we could select a small number of patients, up to 10 in the first instance, who were older, not necessarily suffering from RHD, but who urgently needed heart valve replacement since they would otherwise die shortly, and who were not candidates for conventional open-heart surgery because of their general condition. The inclusion and exclusion criteria were extremely strict. Although the procedures to obtain this approval have been so protracted, and finding acceptable patients so difficult, I could not argue this decision on ethical grounds. The separate SAHPRA process has also been an extensive iterative process, but one that is largely based on thorough documentation of the risk-benefit analysis, as one might expect from a regulatory body that does not wish to make a mistake, especially this early in their existence.
Now, in November 2023, subject to some final documentation and the agreement of our FiM patient and his/her clinical team, we are primed to go.
The Next Steps
We did not form SAT in order to develop one product that would be eventually tested in 10 non- RHD patients. Our objectives were to generate a technology platform that would be the basis of a global impact on RDH patients, first with the bioprosthetic valve and, soon after, with a polymeric valve. It is clear to us that once we have shown that the bioprosthetic valve can be effectively implanted and extends the functional life of RHD patients, we will be in a strong position to move forward.
We are now facing the realities of scaled-up manufacturing and commercialization, neither of which are strong features of the company. Because of the need for labor-intensive stitching, the bioprosthetic valve may still only have a niche market in the BRICS countries, but the polymer valve will be much more amenable for mass production. This valve, as shown, is now fully developed and will undergo extensive animal trials starting very soon, with an eye on SAHPRA submission at the end of 2024. Since this is likely to be the world’s first polymeric TAVR, the global interest should be very significant.
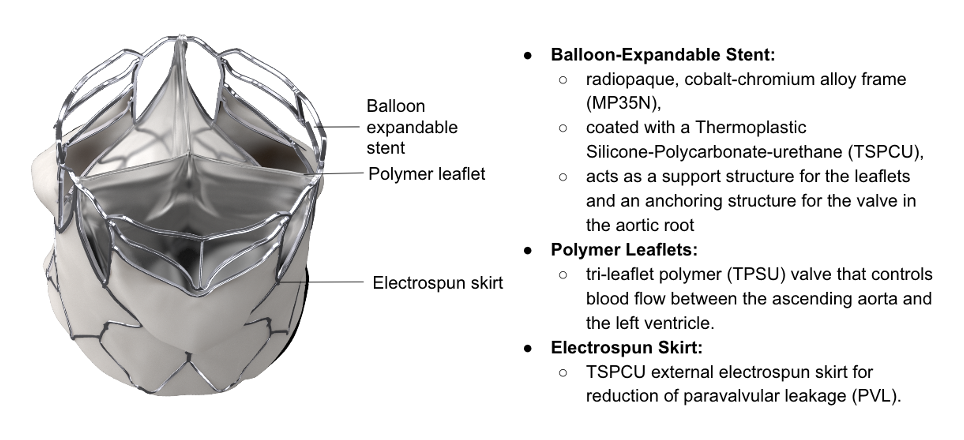
As the supply chain and skilled engineering staff issues have shown, SAT may not be in the best position to scale-up in this way, nor to market the valves in all relevant regions of the world. It is our intention to partner with one or more global medical device companies over the next year, so that we can fully realize the clinical benefit of our inventions, while at the same time producing a good return for our invaluable investors.

